IMPORTING MEDICAL RECORDING PAPER INTO THE UNITED KINGDOM CAN BE EASY

The United Kingdom’s (UK) departure from the European Union had changed the importing process of medical recording paper into the UK (England, Wales, Scotland and Northern Ireland). The changes are in the product registration process, customs document requirement, product conformity/certification, and the authorized representative.
All medical products declared as medical devices need to comply with the UK Medicines and Healthcare Products Regulatory Agency (MHRA). It is responsible for regulating the UK medical device market through certification, conformity marking and registration. Also, a UK Responsible person needs to be appointed by the manufacturer outside of the UK, before the medical device can enter into the UK market. However, different rules apply to Northern Ireland.
Tele-Paper’s Medical Recording Paper Is Registered with the UK Medicines and Healthcare products Regulatory Agency (MHRA)
The UK Medicines and Healthcare Products Regulatory Agency (MHRA) is the regulatory body responsible for ensuring the safety, quality, and effectiveness of medical devices and medicines in the UK. Importing medical recording paper into the UK can be a complicated process, especially if the manufacturer is based outside the UK. However, we have set certain measures in place that can make the process of purchasing from us easier for you.
If you are importing medical recording paper, fetal monitoring recording paper (CTG paper), or electrocardiogram paper (ECG paper), you can rest easy knowing that our products are already registered with the MHRA. This shows that we have met the requirements and standards set by the agency. This will eliminate the extra steps that you and other manufacturers would have to take for you to be able to import the paper. To know more about the role of UK MHRA, click here.
We Have a UK Responsible Person
In addition to being registered with the MHRA, we have also appointed a UK Responsible Person. Since the UK is no longer a part of the European Union, The UK Responsible Person plays an important role in ensuring compliance with the regulatory requirements for medical devices. Previously, this role was done by the EU Authorised Representative.
The UK Responsible Person is responsible for tasks such as registering the medical devices with the MHRA, maintaining the technical documentation, and ensuring that the medical device meets the requirements. Some of their responsibility includes:
- Ensure that the manufacturer has prepared the declaration of conformity and technical documentation, and if required, has performed the necessary conformity assessment procedure.
- Keep copies of the technical documentation, declaration of conformity, and relevant certificate available for inspection by the MHRA.
- Provide the MHRA with all the information and documentation needed to prove the device’s conformity upon request.
- If available, comply with any MHRA request to provide samples.
- And more.
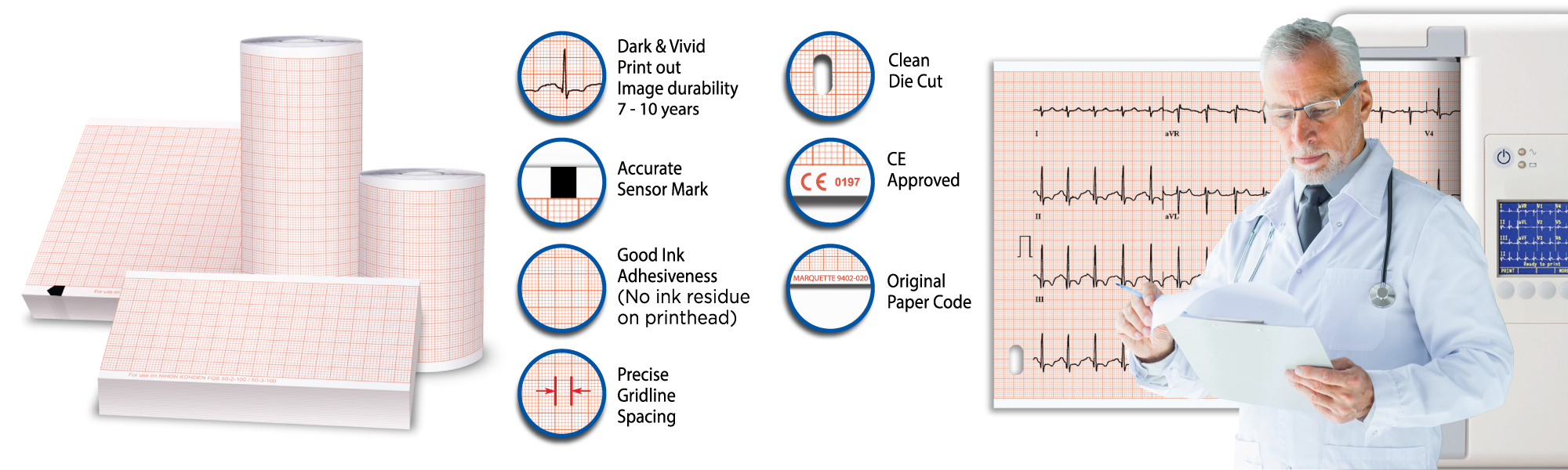
Tele-Paper’s Medical Recording Paper
We manufacture and export over 250 types of medical recording paper. Saving you costs by importing from one place.
A reputable brand, reasonable price, convincing paper quality, premium service and consistent supply is our slogan.
Our brand is preferred by healthcare professionals worldwide due to:
- Long-lasting image around 7 – 10 years. Important for documentation regulation.
- Excellent linear tearing.
- Machine-friendly with international standard design, preventing jamming and other problems to the machine.
With our paper mill, you can expect a reasonable price and consistent supply even during the peak holiday season. To know more about our medical recording paper, visit our product page, or click here.

TELEPAPER MANUFACTURE AND EXPORT MEDICAL RECORDING PAPER INTO UNITED KINGDOM
We have been manufacturing and exporting premium papers globally for over 30 years.
When importing Medical Recording Paper from us into England, Wales, Scotland or Ireland, we have set certain measures to ensure that the process is as smooth as possible. By having our papers registered with MHRA and having appointed a UK Responsible Person to act on our behalf. This will make your job in importing the paper easier.
Contact Us
If you’d like to enquire more about our medical paper, send us your enquiry. Or test it yourself, contact us for a free sample.
